Timmerberg's project "Liquid Hydrogen Import in LNG Terminals" addresses three main issues namely identifying technical barriers, outlining appropriate technical solutions and investigating the systemic consequences of switching to liquid hydrogen. First, the materials used have to be examined. "The inner walls of conventional LNG tanks are made of nickel steel, which is unsuitable for use at liquid hydrogen temperatures," said Timmerberg. Possible solutions could be a different inner tank or making the nickel steel hydrogen-tight. The third focus is on the systemic consequences of an LNG terminal's functions: landfall, storage and regasification of LNG are designed to meet the needs of suppliers, customers and terminal operators. "The framework will change with the switch to liquid hydrogen," he added. The regasification of LNG into gaseous natural gas is just one example. "Much of the LNG stored there is vaporised and compressed to provide natural gas for the gas grid. However, when it comes to liquid hydrogen, Europe has only local networks into which gaseous hydrogen could be stored. Liquid hydrogen has benefits for consumers. Thus, integrating a liquid hydrogen terminal into the energy system must be designed distinct from an LNG terminal."
Terminals for importing liquid natural gas (LNG) terminals are going higher up the agenda nowadays as Germany strives to secure its energy supply based on the LNG Acceleration Act. However, natural gas is a fossil fuel and that puts a spanner in the works when it comes to achieving zero greenhouse gas emissions by 2045. "Germany must become independent of fossil fuels to protect the climate and secure the energy supply," according to the German government. "Hydrogen plays a key role therein as it substitutes natural gas, oil and coal. The German government is promoting this promising energy carrier as part of the National Hydrogen Strategy." Plans are also being laid to convert LNG terminals to liquid hydrogen. However, Professor Sebastian Timmerberg, Department of Environmental Engineering at HAW Hamburg, pointed out: "Liquid hydrogen and LNG have significant physical differences. The storage temperature of liquid hydrogen, which is about 90°C lower, poses challenges for LNG terminals." Timmerberg specialises in environmental process engineering, renewable fuels and energy economics and is now researching a concept for converting LNG terminals to liquid hydrogen as part of the Calls for Transfer (C4T) scheme funded by the Hamburg Authority for Science, Research Equality and Districts (BWFGB).
Liquid-Hydrogen Import in LNG Terminals

Developing concept for single-step dimethyl ether synthesis from CO2 and green hydrogen
Hydrogen can be converted into electricity and heat thanks to fuel cells. Yet, the reverse also applies as electricity is needed to produce hydrogen. Green hydrogen uses renewable energies such as photovoltaics or wind power, the extent of which fluctuates depending on sunshine or wind. Thus, storage becomes difficult. Dr Maximilian J. Poller, Institute of Technical and Macromolecular Chemistry at the University of Hamburg, stressed: “That disadvantage can be compensated for by using chemical energy storage concepts as 'surplus' energy can be used to generate hydrogen.” He added: "The green hydrogen obtained in this way can be used to produce chemical energy carriers such as dimethyl ether (DME)."
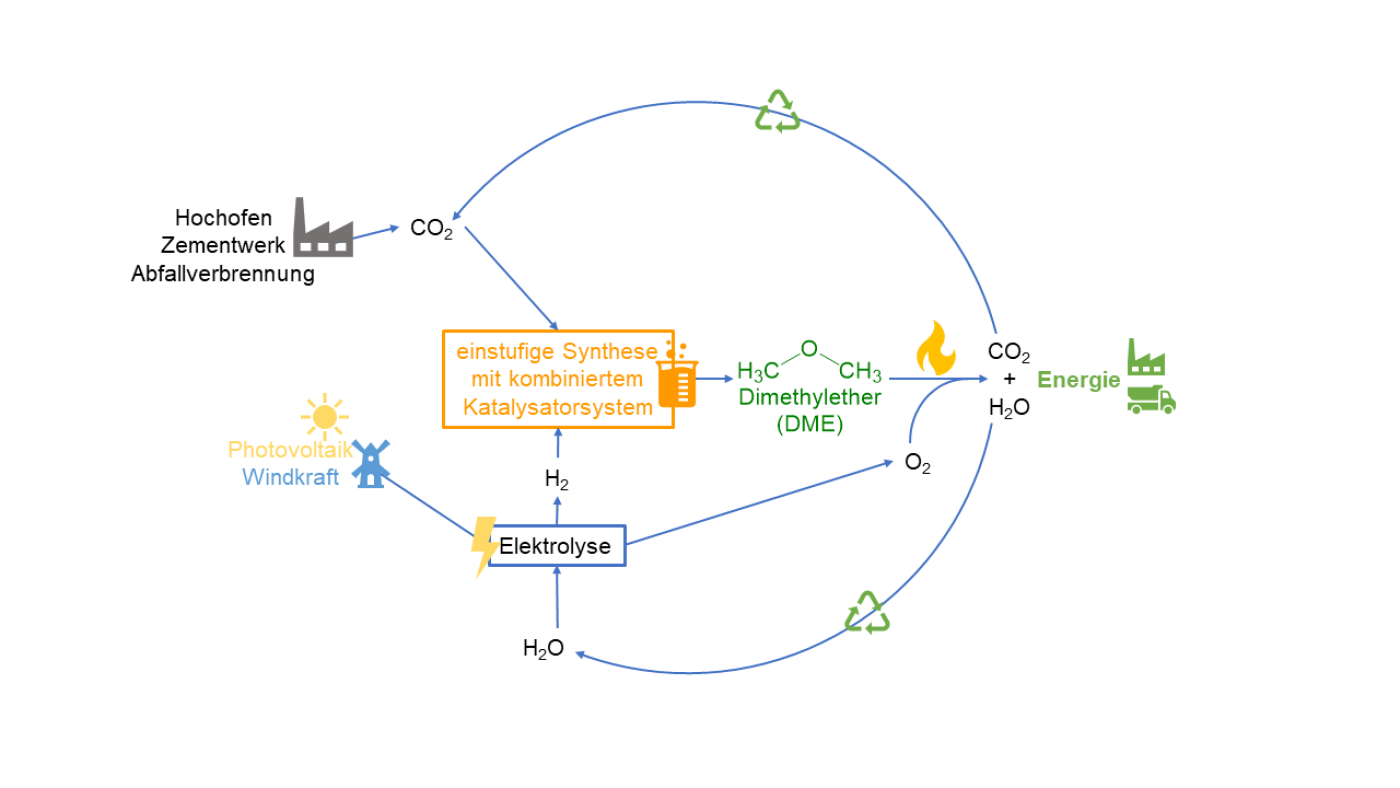
DME as a sustainable fuel variant
DME can be used as e-fuel, which could facilitate the use of internal combustion engines in future. Under new EU law, only new cars with internal, zero-emission combustion engines that are powered by climate-neutral fuels or e-fuels can be registered in the bloc from 2035. The combustion of DME emits less particulate matter, sulfur and nitrogen oxides than conventional diesel fuel. The sustainable fuel is definitely interesting for construction machinery or agricultural and forestry vehicles, Poller noted, and for "vehicles or emergency power generators in disaster control" as well. However, the conventional method of producing DME is not compatible with its use as a storage molecule for green hydrogen. The process is instead energy-intense and many steps are needed to produce DME from fossil raw materials." Commenting on the objective of his project, “Developing a concept for single-step dimethyl ether synthesis from CO2 and green hydrogen,” Poller noted: “We want to solve these problems by applying a new catalyst concept.” The project involves “combining known methanol synthesis catalysts suitable for CO2 with acid catalysts required for the formation of DME." That leads to a sustainable and environment-friendly process for storing chemical energy.
Developing high-pressure dense motion unit for a profile reactor
Another C4T-funded project "Development of a High-Pressure Density Motion Unit for a Profile Reactor" deals with catalysts. "In 90 per cent of all large-scale processes, catalysts are used in industry to accelerate chemical reactions and steer them in a certain direction," says Professor Raimund Horn, who together with Dr Oliver Korup at the Technical University of Hamburg, researches catalytic reactors in which chemical reactions take place on an industrial scale. "Reactors are usually built of steel to withstand high temperatures and pressures. But we can't see inside," said Horn. However, exactly that would be desirable as developing new catalysis processes can prove a real headache.
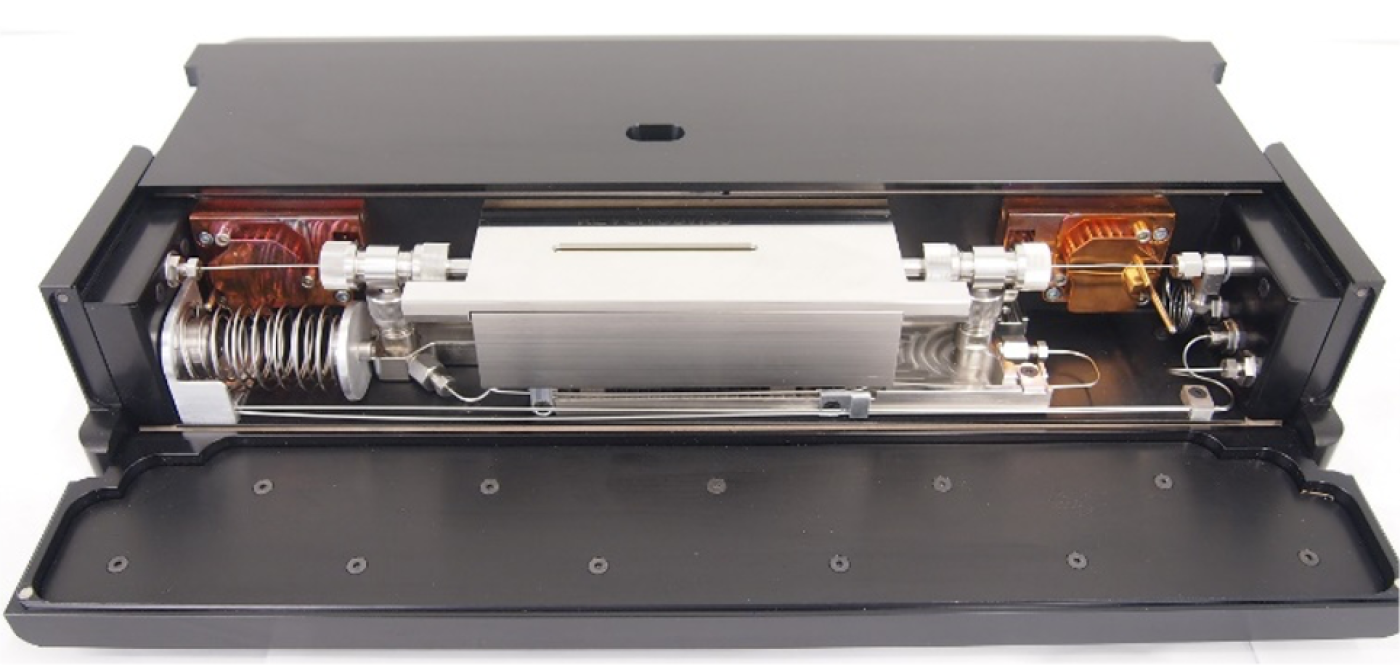
Which molecules are where in the reactor?
The scientists want to use a capillary technique to investigate and optimise catalytic processes. "Our capillary is a thin tube made of steel or glass with a tiny hole in the wall through which we can take a sample from the reactor. In an analyser, we then learn more about the composition of the reaction mixture: which molecules are contained where in the reactor and in what concentration," said Horn. The capillary moves through the reactor from the entrance to the exit to obtain results and is exposed to the internal pressure. "Many of the processes relevant to the energy transition happen under great pressure. Methanol synthesis requires 70 bar, ammonia synthesis even 350 bar." However, reliable measurements have so far only been possible up to 50 bar. Horn and Korup want to change that with a new sealing system. They have set themselves an interim goal of 100 bar and their research has already passed the test. The duo has also founded Reacnostics GmbH to develop, build and sell profile reactors, among others.
Electrolysis systems for river hydropower plants
Hydropower has been used to generate electricity since the 19th century. "However, the potential of flowing water has not been fully used so far, as suitable locations are frequently far away from supply infrastructures and the plants have to react to fluctuating water levels," said Professor Torsten Birth, who specialises in plant engineering and process simulation in energy technology at HAW Hamburg. The River Flow Electrolysis System Platforms (RFESP) project could change that. "As part of RFESP project, a river-based test bed platform for electrolysis systems will be developed and used to demonstrate energy systems." The project is divided into five work packages including the analysis of the technical requirements, developing a concept, validation through a simulation environment and setting up a test platform.

Researching decentralised supply solutions using power-to-gas
"The aim is to obtain realistic research results based on energy system models to explore decentralised supply solutions using power-to-gas," said Birth, who researches the planning and operation of demand-responsive power-to-X and energy systems. "The added value of the project lies in strengthening research, the education and training of specialists, and assisting local industry with the introduction of new technologies and creating jobs."
ys/pb
Read the other parts in our series:
1) Calls for Transfer scheme funding tiny implants for cancer diagnostics
2) Calls for Transfer focuses on recycling chemicals for circular economy
Sources and further information
Calls for Transfer
"Calls for Transfer" is funded by Hamburg's Ministry of Science, Research, Equality and Districts and is sponsored by the University of Technology (TUHH). The project is managed by Hamburg Innovation the board of which makes independent decisions. Hamburg Innovation GmbH is a private knowledge and technology transfer institution at Hamburg's public universities. At the interface between universities, companies and the public sector, the team networks entrepreneurial and scientific potential thereby creating sustainable value for science, politics, business and society.
More
Similar articles

Port of Hamburg to get Germany's first green ammonia terminal

25 hydrogen taxis now on roads across Hamburg

